You can learn 32+ pages from the following data the activation energy for the reaction explanation in Doc format. 1In this equation k is the rate constant for the reaction Z is a proportionality constant that varies from one reaction to another E a is the activation energy for the reaction R is the ideal gas constant in joules per mole kelvin and T is the temperature in kelvin. From the following data plot calculate the activation energyE a for the reaction. In order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature energy of the system. Read also data and from the following data the activation energy for the reaction H2 I2 2HI T K 1T K-1 log10K 769 13 10-3 29667 15 10-3 101.
The activation energy for the reaction calmol HI 1 2HI T in K 1T in K. H 2 I 2 2 H I.

The Activation Energy Of Chemical Reactions When we construct a graph of.
| Topic: From the following data. The Activation Energy Of Chemical Reactions From The Following Data The Activation Energy For The Reaction |
| Content: Answer Sheet |
| File Format: DOC |
| File size: 725kb |
| Number of Pages: 24+ pages |
| Publication Date: September 2019 |
| Open The Activation Energy Of Chemical Reactions |
 |
The activation energy for the reaction calmol H 2 I 2 2HI.

Arrhenius Plot for a Chemical Reaction 300 -550 310 320 330 340 T -600 -650 -700 -750 -800 -850 -900 -950 -1000 1TK x 103 In k. Because the reverse reactions activation energy is the activation energy of the forward reaction plus H of the reaction. 21Temperature is directly related the the kinetic energy of molecules and activation energy Ea is the minimum energy required for a reaction to occur and doesnt change for a reaction. The activation energy for the reaction calmol H 12 2HI Tin K 1Tin K 769 13x10 667 15x10 logK 29 1 4 x 104 3 8 x 104 2 2 x 104 4 3 x 104. We therefore start by calculating 1T and the natural logarithm of the rate constants. The activation energy forthe reaction Calmol H I 2HIT in K7696671Tin K13x1015x10logik29111 4 x 1043 8 x 1042 2 x 1044 3 x 104.
The Chemistry Of Building Better Habits James Clear Energy Activities Chemistry Good Habits Increasing the temperature increases the kinetic energy of the reactants meaning the reactants will move faster and collide with each other more frequently.
| Topic: From the following data the activation energy for the reaction is calmol. The Chemistry Of Building Better Habits James Clear Energy Activities Chemistry Good Habits From The Following Data The Activation Energy For The Reaction |
| Content: Explanation |
| File Format: DOC |
| File size: 3.4mb |
| Number of Pages: 30+ pages |
| Publication Date: December 2017 |
| Open The Chemistry Of Building Better Habits James Clear Energy Activities Chemistry Good Habits |
 |
Enzymes Allow Activation Energies To Be Lowered Learn Science At Scitable From the following data the activation energy for the reaction c a l m o l is.
| Topic: From the following data. Enzymes Allow Activation Energies To Be Lowered Learn Science At Scitable From The Following Data The Activation Energy For The Reaction |
| Content: Summary |
| File Format: Google Sheet |
| File size: 2.2mb |
| Number of Pages: 30+ pages |
| Publication Date: December 2021 |
| Open Enzymes Allow Activation Energies To Be Lowered Learn Science At Scitable |
 |

Activation Energy 1632 Determine activation energy Ea values from the Arrhenius equation by a graphical methodNote that in the exam you will be given the graph already p.
| Topic: Chemistry QA Library From the following data plot calculate the activation energy E for the reaction. Activation Energy From The Following Data The Activation Energy For The Reaction |
| Content: Synopsis |
| File Format: PDF |
| File size: 800kb |
| Number of Pages: 13+ pages |
| Publication Date: August 2021 |
| Open Activation Energy |
 |

Enzymes And The Active Site Article Khan Academy We therefore start by calculating 1T and the natural logarithm of the rate constants.
| Topic: The activation energy for the reaction calmol H 12 2HI Tin K 1Tin K 769 13x10 667 15x10 logK 29 1 4 x 104 3 8 x 104 2 2 x 104 4 3 x 104. Enzymes And The Active Site Article Khan Academy From The Following Data The Activation Energy For The Reaction |
| Content: Explanation |
| File Format: PDF |
| File size: 1.5mb |
| Number of Pages: 35+ pages |
| Publication Date: January 2019 |
| Open Enzymes And The Active Site Article Khan Academy |
 |

Activation Energy And Temperature Dependence Chemistry Master
| Topic: Activation Energy And Temperature Dependence Chemistry Master From The Following Data The Activation Energy For The Reaction |
| Content: Explanation |
| File Format: Google Sheet |
| File size: 1.4mb |
| Number of Pages: 50+ pages |
| Publication Date: December 2019 |
| Open Activation Energy And Temperature Dependence Chemistry Master |
 |
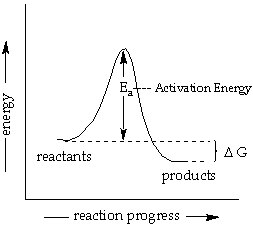
Label The Energy Diagram For A Two Step Re Clutch Prep
| Topic: Label The Energy Diagram For A Two Step Re Clutch Prep From The Following Data The Activation Energy For The Reaction |
| Content: Answer |
| File Format: Google Sheet |
| File size: 725kb |
| Number of Pages: 20+ pages |
| Publication Date: January 2020 |
| Open Label The Energy Diagram For A Two Step Re Clutch Prep |
 |

Activation Energy And Temperature Dependence Of Rates According To Collision Theory Molecules Must Collide To React At A Higher Temperature Molecules Move Faster And Will Collide More Often Also At A Higher Temperature The Reactant Molecules Carry
| Topic: Activation Energy And Temperature Dependence Of Rates According To Collision Theory Molecules Must Collide To React At A Higher Temperature Molecules Move Faster And Will Collide More Often Also At A Higher Temperature The Reactant Molecules Carry From The Following Data The Activation Energy For The Reaction |
| Content: Answer Sheet |
| File Format: Google Sheet |
| File size: 810kb |
| Number of Pages: 5+ pages |
| Publication Date: May 2017 |
| Open Activation Energy And Temperature Dependence Of Rates According To Collision Theory Molecules Must Collide To React At A Higher Temperature Molecules Move Faster And Will Collide More Often Also At A Higher Temperature The Reactant Molecules Carry |
 |

If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Pare With The Activation Energy For The Reverse Reaction B A Socratic
| Topic: If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Pare With The Activation Energy For The Reverse Reaction B A Socratic From The Following Data The Activation Energy For The Reaction |
| Content: Explanation |
| File Format: DOC |
| File size: 800kb |
| Number of Pages: 21+ pages |
| Publication Date: July 2021 |
| Open If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Pare With The Activation Energy For The Reverse Reaction B A Socratic |
 |

18 4 Potential Energy Diagrams Chemistry Libretexts
| Topic: 18 4 Potential Energy Diagrams Chemistry Libretexts From The Following Data The Activation Energy For The Reaction |
| Content: Answer |
| File Format: DOC |
| File size: 1.4mb |
| Number of Pages: 7+ pages |
| Publication Date: September 2021 |
| Open 18 4 Potential Energy Diagrams Chemistry Libretexts |
 |

Activation Energy
| Topic: Activation Energy From The Following Data The Activation Energy For The Reaction |
| Content: Answer Sheet |
| File Format: PDF |
| File size: 1.9mb |
| Number of Pages: 50+ pages |
| Publication Date: October 2020 |
| Open Activation Energy |
 |

S Nfschools Cms Lib Ny19000301 Centricity Domain 2312 5 4 20 205 8 20answers Pdf
| Topic: S Nfschools Cms Lib Ny19000301 Centricity Domain 2312 5 4 20 205 8 20answers Pdf From The Following Data The Activation Energy For The Reaction |
| Content: Learning Guide |
| File Format: PDF |
| File size: 1.6mb |
| Number of Pages: 23+ pages |
| Publication Date: October 2019 |
| Open S Nfschools Cms Lib Ny19000301 Centricity Domain 2312 5 4 20 205 8 20answers Pdf |
 |
Its really easy to prepare for from the following data the activation energy for the reaction Activation energy and temperature dependence chemistry master enzymes allow activation energies to be lowered learn science at scitable s nfschools cms lib ny19000301 centricity domain 2312 5 4 20 205 8 20answers pdf activation energy and temperature dependence of rates according to collision theory molecules must collide to react at a higher temperature molecules move faster and will collide more often also at a higher temperature the reactant molecules carry the activation energy of chemical reactions activation energy enzymes and the active site article khan academy the activation energy of chemical reactions